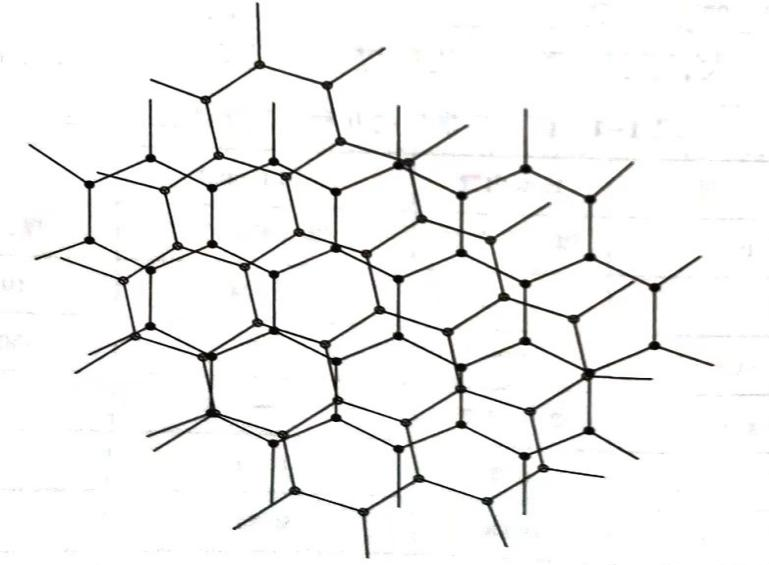
The atomic structure of coal-based activated carbon is a chaotic layer structure containing graphite microcrystals.
The so-called graphite microcrystalline disordered layer structure is similar to the two-dimensional structure of graphite, but its structure is different from that of graphite, in that the parallel layers are not completely oriented with respect to their common vertical axis, and the planes overlap each other irregularly, thereby forming a disordered layer arrangement structure.
The most common basic crystallites consist of about 3~4 parallel graphite laminations, whose diameter is about 9 times the width of a carbon hexagon. The size of the basic crystallites is often increased by the increase of activation or carbonization temperature. The pores are formed by the interstices between the graphitic crystallites.
The crystallites are different in size and the crystallite structure is not uniform, which gives the activated carbon macroadsorption ability. In addition, in addition to carbon, there are other elements such as nitrogen, oxygen and hydrogen, which exist in the form of surface functional groups, resulting in different surface chemical properties of activated carbon.